Acidification
This Fact Page displays text and images related to global warming and climate change
(Hover your mouse over the text below to "popup" a window with a related text.
Click on the text or image to open a new window with a detailed description.)
|
|
| Ocean pH Since 1850 and Projected to 2100 | 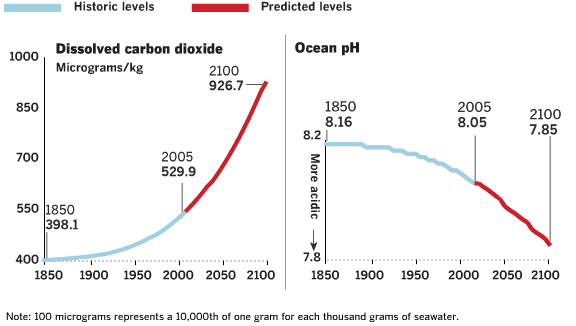
Based on "Ocean acidification due to atmospheric carbon dioxode, Altered Oceans: A Chemical Imbalance". See also "Ocean acidification due to increasing
atmospheric carbon dioxide" (http://www.us-ocb.org/publications/Royal_Soc_OA.pdf) | Good source for ocean acidification information: http://news-oceanacidification-icc.org/ | | | Source: theoildrum | | URL: http://www.theoildrum.com/node/4818 |
| Ocean pH vs Atmospheric CO2 PPM | 
Table 1. Changes to ocean chemistry and pH estimated using the OCMIP3 models calculated from surface ocean
measurements and our understanding of ocean chemistry. Note that the concentration of bicarbonate ion (HCO3
–
) and
carbonic acid (H2CO3) increase with rising atmospheric concentration of CO2 while carbonate ion (CO3
2–) decreases. The
average pH of the surface ocean waters decreases with increasing atmospheric CO2 concentration. (Assumptions used
in model: Total alkalinity = 2324 mol/kg, temperature = 18° C. All other assumptions as per OCMIP3 (Institut Pierre
Simon Laplace 2005). Aragonite and calcite saturation calculated as per Mucci & Morse (1990). Physical oceanographic
modelling is based on Bryan (1969) and Cox (1984). | If CO2 emissions continue on current trends, this could
result in the average pH of the surface oceans
decreasing by 0.5 units below the level in pre-industrial
times, by 2100. This is beyond the range of natural
variability and represents a level probably not
experienced for at least hundreds of thousands of years
and possibly much longer (Caldeira & Wickett 2003).
Critically, the rate of change is also at least 100 times
higher than the maximum rate observed during this time
period. These changes are so rapid that they will
significantly reduce the buffering capacity of the natural
processes that have moderated changes in ocean
chemistry over most of geological time. | | | Source: Royal Society | URL: http://www.us-ocb.org/publications/Royal_Soc_OA.pdf
(The text for the image(s) on this Web page was taken from the above source.) |
| Ocean pH vs Atmospheric ppm 1958 to 2012 | | As a result of the increase in atmospheric CO2, the oceans have become more acidic | 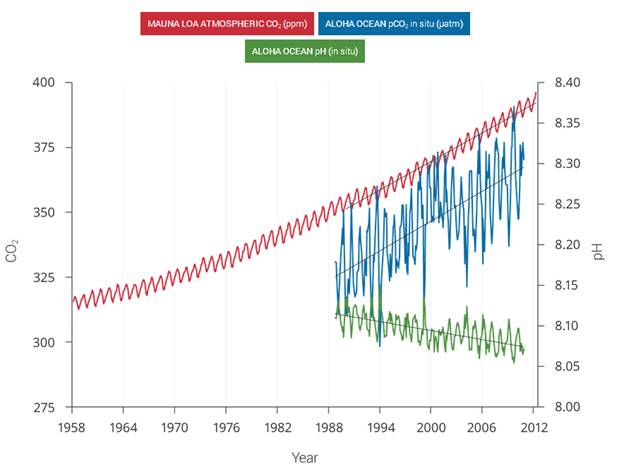
Figure 2.30: The correlation between rising levels of CO2 in the atmosphere (red) at Mauna Loa and rising CO2 levels (blue) and falling pH (green) in the nearby ocean at Station Aloha. As CO2 accumulates in the ocean, the water becomes more acidic (the pH declines). (Figure source: modified from Feely et al. 2009). | As human-induced emissions of carbon dioxide (CO2) build up in the atmosphere, excess CO2 is dissolving into the oceans where it reacts with seawater to form carbonic acid, lowering ocean pH levels (“acidification”) and threatening a number of marine ecosystems. Currently, the oceans absorbs about a quarter of the CO2 humans produce every year. Over the last 250 years, the oceans have absorbed 560 billion tons of CO2, increasing the acidity of surface waters by 30%.,, Although the average oceanic pH can vary on interglacial timescales, the current observed rate of change is roughly 50 times faster than known historical change., Regional factors such as coastal upwelling, changes in discharge rates from rivers and glaciers, sea ice loss, and urbanization have created “ocean acidification hotspots” where changes are occurring at even faster rates. The acidification of the oceans has already caused a suppression of carbonate ion concentrations that are critical for marine calcifying animals such as corals, zooplankton, and shellfish. Many of these animals form the foundation of the marine food web. Today, more than a billion people worldwide rely on food from the ocean as their primary source of protein. Ocean acidification puts this important resource at risk.
Observations have shown that the northeastern Pacific Ocean, including the Arctic and sub-Arctic seas, is particularly susceptible to significant shifts in pH and calcium carbonate saturation levels. Recent analyses show that large areas of the oceans along the U.S. west coast,, the Bering Sea, and the western Arctic Ocean, will become difficult for calcifying animals within the next 50 years. In particular, animals that form calcium carbonate shells, including corals, crabs, clams, oysters, and tiny free-swimming snails called pteropods, could be particularly vulnerable, especially during the larval stage.,,, | | | Source: National Climate Assessment | URL: http://nca2014.globalchange.gov/report/our-changing-climate/ocean-acidification
(The text for the image(s) on this Web page was taken from the above source.) |
| National Academy of Sciences - Climate Change: Evidence and Causes | | What is ocean acidification and why does it matter? | 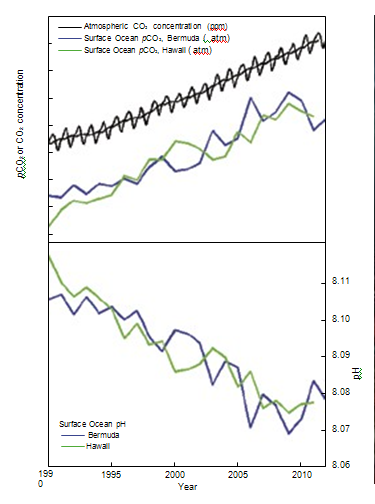
figure 7. As CO2 in the air has
increased, there has been an
increase in the CO2 content of the
surface ocean (upper box), and a
decrease in the seawater pH (lower
box). Source: adapted from Dore et al.
(2009) and Bates et al. (2012). | Direct observations of ocean chemistry have shown that the chemical balance of seawater
has shifted to a more acidic state (lower pH) [Figure 7]. Some marine organisms (such
as corals and some shellfish) have shells composed of calcium carbonate which dissolves
more readily in acid. As the acidity of sea water increases, it becomes more difficult for
them to form or maintain their shells. CO2 dissolves in water to form a weak acid, and the oceans have absorbed about a third of the CO2 resulting
from human activities, leading to a steady decrease in ocean pH levels. With increasing atmospheric CO2,
the chemical balance will change even more during the next century. Laboratory and other experiments
show that under high CO2 and in more acidic waters, some marine species have misshapen shells and
lower growth rates, although the effect varies among species. Acidification also alters the cycling of
nutrients and many other elements and compounds in the ocean, and it is likely to shift the competitive
advantage among species, with as-yet-to-be-determined impacts on marine ecosystems and the food web. | | | Source: NAS | URL: http://dels.nas.edu/resources/static-assets/exec-office-other/climate-change-QA.pdf
(The text for the image(s) on this Web page was taken from the above source.) |
|
|